Abstract
INTRODUCTION: Pathogen inactivation (PI) of platelet (PLT) concentrates shows good efficacy against a broad array of viruses, bacteria and parasites. Moreover, animal studies have shown that the technology reduces alloimmunization due to HLA alloantibody formation. As bleeding is considered to be the pivotal outcome for PLT transfusion trials, we conducted a trial comparing PI-treated PLT concentrates using the Mirasol technology (Terumo BCT, Lakewood, CO) with standard untreated PLT concentrates, with the percentage of transfusion episodes in which a > grade 2 bleeding complication (World Health Organization grading) occurred as primary outcome.
METHODS: The PREPAReS study was designed as a randomized multicenter non-inferiority study using a parallel arm design. Patients aged 18 years or older were eligible if they were expected to require at least two PLT transfusions. Exclusion criteria included: WHO bleeding grade > 2 at randomization, known immunological refractoriness to platelet transfusions, indications to use hyperconcentrates, prior treatment with pathogen-reduced blood products, pregnancy, microangiopathic thrombocytopenia, ITP, and known allergy to riboflavin or its photoactive products. Patients were assigned to the Control group receiving standard plasma-stored PLT concentrates, or the Study group receiving Mirasol-treated PLTs. Patients could be re-enrolled, thereby adding multiple transfusion episodes to the trial. A Transfusion Episode was defined as the time from randomization until the time the patient went off trial. PLT concentrates were prepared from pooled buffy coats, resuspended in plasma and leukoreduced by filtration. For pathogen reduction, 35 ml (500 µM) riboflavin was added within 8 hours of preparation of the platelets, and exposed to UV light. PLT products were stored with gentle agitation at 20-24°C up to five days in Canada and for a maximum of seven days in the Netherlands and Norway. The Intention To Treat (ITT) analysis included all bleeding episodes from the moment of randomization on, the Per Protocol (PP) analysis included only bleeding episodes that occurred after the first platelet transfusion. Patients who were actively bleeding on the day of the first transfusion, or received more than 25% off-protocol transfusions were excluded. The PREPAReS trial was powered to demonstrate non-inferiority in the ITT analysis.
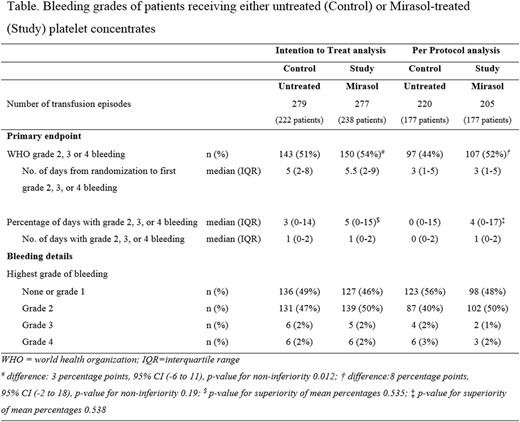
RESULTS: Between November 2010 and April 2016, 567 transfusion episodes were randomized (283 in the Control group and 284 in the Study group; 469 unique patients), of which 11 were excluded due to active bleeding at the time of randomization, or due to gross incompliance. For the PP analysis, 37 episodes were excluded due to active bleeding at the time of the first platelet transfusion (Control, 21, Study, 16), because the patient did not receive a platelet transfusion, or because more than 25% were off-protocol transfusions (Control, 38, Study, 56), rendering 425 episodes evaluable. The ITT and PP outcomes are shown in the Table. The primary endpoint was met in the ITT analysis, but not in the PP analysis.
CONCLUSION: For Mirasol-treated platelets compared with untreated platelets with bleeding as primary study outcome, the non-inferiority criterion was met in the ITT analysis, but not in the PP analysis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal